New era of human embryo gene editing begins
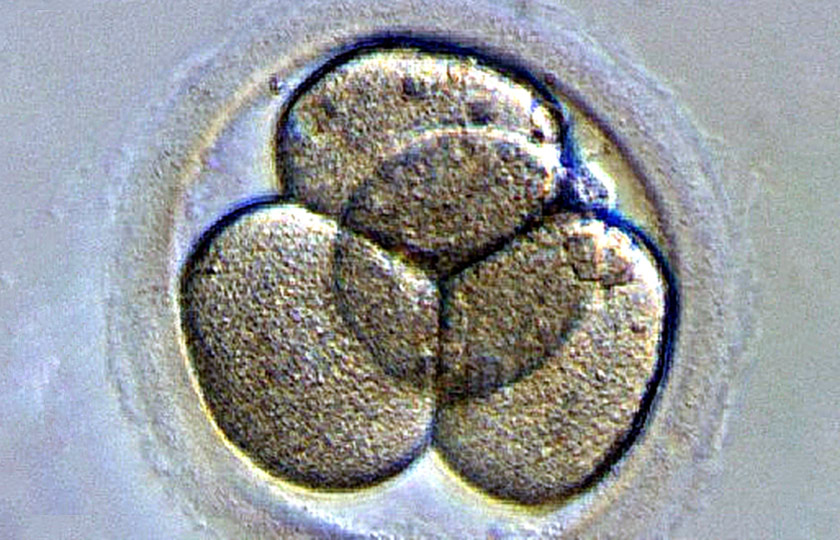
A Swedish scientist is gene editing healthy human embryos, and he is probably not alone, researchers say.
Chinese researchers have twice reported editing genes in human embryos that are unable to develop into a baby (SN Online: 4/6/16; SN Online: 4/23/15). But developmental biologist Fredrik Lanner of the Karolinska Institute in Stockholm is the first researcher to publicly acknowledge editing genes in viable human embryos. Other researchers are almost certainly doing similar experiments out of the public eye, scientists say.
“My sense is that there are different groups out there doing this kind of work, but they haven’t opened up their labs” to reporters, says stem cell biologist Paul Knoepfler at the University of California, Davis.
Lanner allowed a reporter from National Public Radio exclusive access to watch as researchers in his lab injected early human embryos left over from in vitro fertilization with a powerful new gene editor called CRISPR/Cas9 (SN: 9/3/16, p. 22). The editor is a two-part molecular scissors consisting of a DNA-cutting protein called Cas9 and a short piece of RNA that guides the protein to a gene that scientists want to snip. The technology allows researchers to easily make precise cuts in DNA, something that has been tricky to do in humans before.
CRISPR’s ease and precision have made the prospect of editing human embryos nearly inevitable, says Chad Cowan, a stem cell biologist at Harvard University. “Now that you can do that and garner considerable attention, it’s basically light to moths,” he says. “There will be certain people who will find it irresistible to do it whether it’s scientifically justified or not.”
Such experiments are probably going on privately in the United States, China and other countries, he says. In February, the United Kingdom gave developmental biologist Kathy Niakan of the Francis Crick Institute in London a license to perform gene editing on early human embryos (SN Online: 2/1/16). Those experiments have not yet begun and are undergoing further reviews to make sure Niakan has a “completely ethically tight argument,” Cowan says.
Sweden and other countries do require proper scientific justification and ethical oversight to do such research, Cowan adds, but researchers are often reluctant to talk to reporters before their work has been published in scientific journals. Harvard geneticist George Church confirmed in an e-mail to Science News that Lanner’s experiments are the first to be disclosed publicly, but don’t represent the first time researchers have done gene editing on viable human embryos. There is a growing movement to engage and inform the public prior to conducting such controversial research, Church says, especially experiments involving CRISPR/Cas9.
Lanner is conducting basic research to learn more about the development of early embryos in order to devise new infertility treatments, prevent miscarriages and learn more about stem cells, according to the NPR report. The altered embryos would be discarded after 14 days of growing in a laboratory dish and would not be implanted in a woman’s uterus to establish pregnancy. Such work “can lead to insights which can have big impact clinically and, ironically, won’t require embryo editing,” Church says.
An international summit on human gene editing last year recommended that such fundamental research could and should be done, given proper scientific justification and ethical oversight (SN: 12/26/15, p. 12). But many people worry that such research, especially on viable embryos, opens the door to creating designer babies and forever altering the genetic heritage of humans (SN: 5/30/15, p. 16).



