Scientists need to redraw picture of cell’s biggest organelle
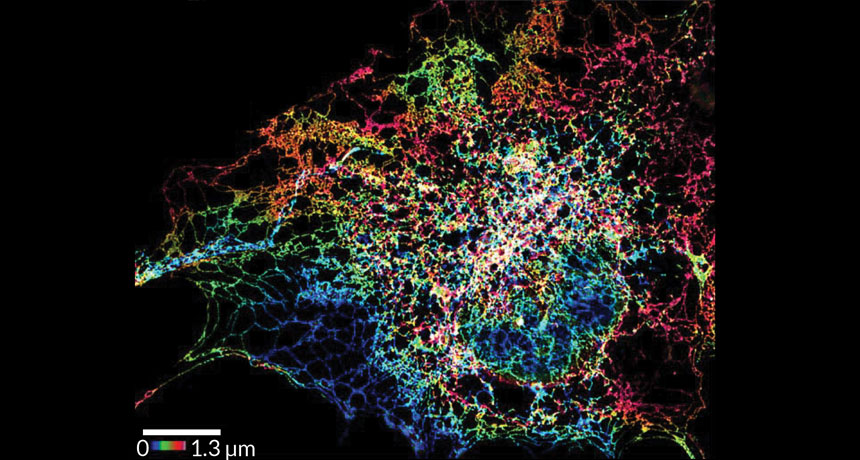
Textbook drawings of the cell’s largest organelle might need to be updated based on new images. Super-resolution shots of the endoplasmic reticulum reveal tightly packed tubes where previous pictures showed plain flat sheets, scientists report in the Oct. 28 Science.
The finding helps explain how the endoplasmic reticulum, or ER, reshapes itself in response to changing conditions, says study coauthor Jennifer Lippincott-Schwartz, a cell biologist at the Howard Hughes Medical Institute’s Janelia Research Campus in Ashburn, Va.
The ER is a snaking network of membranes that stretches from the nucleus of the cell to its edge. A sort of cellular jack-of-all-trades, it provides scaffolding for protein-producing ribosomes and makes sure those proteins are folded properly. It churns out lipids. And it stores and releases calcium, which sends messages within and between cells. Endoplasmic reticulum stress or malfunction can contribute to neurodegenerative diseases like Alzheimer’s and Parkinson’s.
Scientists have peered at this organelle under microscopes many times before. But newer super-resolution microscopy techniques reveal details just tens of nanometers wide, far smaller than what conventional microscopes can see. That resolution upgrade showed that apparently flat sheets of membranes actually consisted of dense clusters of tubules vibrating and shifting.
“A lot of what we’ve assumed based on the tools that we had really isn’t true,” says study coauthor Craig Blackstone, a cell biologist at the National Institute of Neurological Disorders and Stroke in Bethesda, Md. Instead of being made of a mixture of sheets and tubes, the outer region of the ER turns out to be made mostly just of tubes.
Those tiny tubules come together in three-way junctions, linking into a mesh network that resembles a stretchy spider web. When the ER needs to move into a new part of the cell, the tubes can expand or contract. And the junctions can also slide up and down the tubes like curtains on a rod, the team found.
“You can’t pull a sheet apart very easily except by breaking it,” Lippincott-Schwartz says, but the tubes are far more adaptable.
The tubes are packed to different densities throughout the ER, perhaps reflecting the various jobs that different parts of the sprawling organelle take on.
The team still saw bona fide sheets in the part of the ER closest to the cell’s nucleus, a feature other scientists have also reported. Those sheets were stacked on top of each other like pancakes.
“In the past, people have had to use electron microscopes to look at the ER,” says Mark Terasaki, a cell biologist at the University of Connecticut Health Center in Farmington who wasn’t involved in the study. But that requires killing the cells, capturing their inner structure at just one moment in time. These new imaging techniques captured the motion of the ER in living cells, showing how the tubes rapidly vibrated and remodeled themselves into different shapes.
More than a glamour shot, the up-close imaging also gives important context for doctors studying diseases affecting the ER. For instance, Blackstone studies a group of diseases called hereditary spastic paraplegias, which weaken lower limbs and make it hard for people to walk. Mutations in genes that make ER-shaping proteins cause the symptoms in some patients.
“For us to really understand disease, we need to understand what normal is,” says Blackstone. Now, he says, his lab can compare the ER structures of healthy and sick people to figure out exactly what’s going wrong.



